Chapter 7 The Structure of Atoms and Periodic Trends. Choose the larger atom from each of the following pairs.
Foothill Edu

Electron Configuration For Ni Ni2 And Ni3 Nickel And Nickel Ions Bluevelvetrestaurant
25 5 Bonding In Coordinate Compounds Chemistry Libretexts
The electron configuration will display below the diagram.

Ni2+ electron configuration. Chemistry unit 4 worksheet 4 quizlet. Ni 2 P decorated hollow carbon spheres Ni 2 P-HCS were constructed as bi-functional mediator for Li-S batteries. For the electronelectron exchange and.
An electron is moving at 106 ms in a direction parallel to a current of 5 A flowing through an infinitely long straight wire separated by perpendicular distance of 10 cm in air. The outer electronic configuration of the ground state chromium atom is 3d44s2. NCERT Solutions for Class 12 Chemistry Chapter 8 Free PDF Download.
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties. Electron configurations worksheet quia. The valence electronic configuration of Co 2 is 3d 7 with.
Academiaedu is a platform for academics to share research papers. Electron configuration quiz answer key. Nickel is a silvery-white metal with a slight golden tinge that takes a high polish.
1s22s22p6Ne is paired with the IE 2080 kJmol. Lewis is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adductA Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis. 13 Electron Configuration T simontechnology org.
Calculate the magnitude of the force experienced by electron. Ar 4s0 3d8 Ar 4s0 3d5 diamagnetic. Electron Configuration CHEM 1A 1.
Choose the element with the highest first. An electron is contained in a one-dimensional box of length 0370 nm. O2-Br-Sr2 Co3 Cu2 He 2s2 2p6 Ar 3d10 4s2 4p6.
This video explains the answers to the practice quiz on electron configurations. Fe 13 CHe1 14 Ni2 15. Al or In Br or Ar S or Sn Si or Cl.
Mn2s3 anion - kala-namakpl. We first need to find the number of. A Draw an energy-level diagram for the electron for levels up to n 4.
626 10 An excited state of an atom is a state where its potential energy is higher than the ground state. 2eV to reach this zero point W 0. In Br Sn Si.
1982 - 1 Mark 42. To write the configuration for the Nickel ions first we need to write the electron configuration for just Nickel Ni. It becomes Ni2 by losing 2 electrons hence configuration of Ni2 is.
The 1s22s22p6 Ne is a noble gas electron configuration. Valence electrons and ions worksheet. Create the correct electron configuration for argon.
In this example the electron configuration for Ni2 still kept its 3d8 but lost the 4s2 became 4s0 because the s-orbital has the highest energy level of n 4 in this case. Shapes of s p and d orbitals electron spin and spin quantum number. Ar 4s0 3d8 So nickel loses two electrons from the 4s orbital not the 3d orbital as per the Aufbau principle 25.
A Lewis acid named for the American physical chemist Gilbert N. The 2p electron is also no shielded by electron in the same energy level. Complete Solutions Manual General Chemistry Ninth Edition.
Rules for filling electrons in orbitals aufbau. It is one of only four elements that are magnetic at or near room temperature the others being iron cobalt and gadoliniumIts Curie temperature is 355 C 671 F meaning that bulk nickel is non-magnetic above this temperature. Therefore it will be extremely difficult to remove an electron.
The ground state energy of hydrogen atom is 136 eV. Writing Electron Configurations and Counting Valence Electrons Answers 13 KB. These NCERT Solutions for Class 12 Chemistry are updated to the latest term II CBSE Syllabus for 2021-22 and.
As the interlayer Ni 2 P-HCS has strong interaction and efficient electrocatalysis toward polysulfides. A square coil of side 10 cm consists of 20 turns and carries a current of 12 A. Catalytic effectiveness value was proposed to evaluate the.
The energy of the electron in the 3d-orbital is less than that in the 4s-orbital in the hydrogen atom. 1983 - 1 Mark 41. As the Li host lithiated Ni 2 P-HCS mixed ion-electron conductor Li 3 PNi enables regulated Li deposition.
The unit cell of nickel is a face-centered cube with the lattice parameter of. Pdf FREE PDF DOWNLOAD Practice Problems Chapter 8. Elementary ideas of quantum mechanics quantum mechanical model of atom its important features concept of atomic orbitals as one electron wave functions.
In this example the electron configuration for Ni2 still kept its 3d8 but lost the 4s2 became 4s0 because the s-orbital has the highest energy level of n 4 in this case. Write a ground state electron configuration for these ions. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon.
Electron Configuration Practice Chemistry Name. The second electron joins the first in the 1s - orbital so the electron configuration of the ground state of He is 1s 2. Various quantum numbers principal angular momentum and magnetic quantum numbers and their significance.
This configuration is also written as. Is Is 2s 2P. Fe 13 CHe1 14 Ni2 15.
Rt has three extra electrons 11 02 2 12. From hydrated Ni3OH2C8H4O42H2O4 to anhydrous Ni2OH2C8H4O4. Electron configuration gizmo answer key activity c 883 0 obj stream Arrange.
Academiaedu is a platform for academics to share research papers. Remember that ions have a change in the total number of electrons positive have lost electrons and negative have gained. Ground state vs excited state electron configuration worksheet answers The electron configuration of an element is the arrangement of its electrons in its atomic orbitals.
NCERT Solutions for Class 12 Chemistry Chapter 8 The d and f Block Elements is a powerful study material that has answers to textbook exercises and important questions from the previous year and sample papers.

Solved What Is The Ground State Electron Configuration Of An Chegg Com

Nickel Atom Can Lose Two Electrons To Form Ni 2 Ion The Atomic Number Youtube

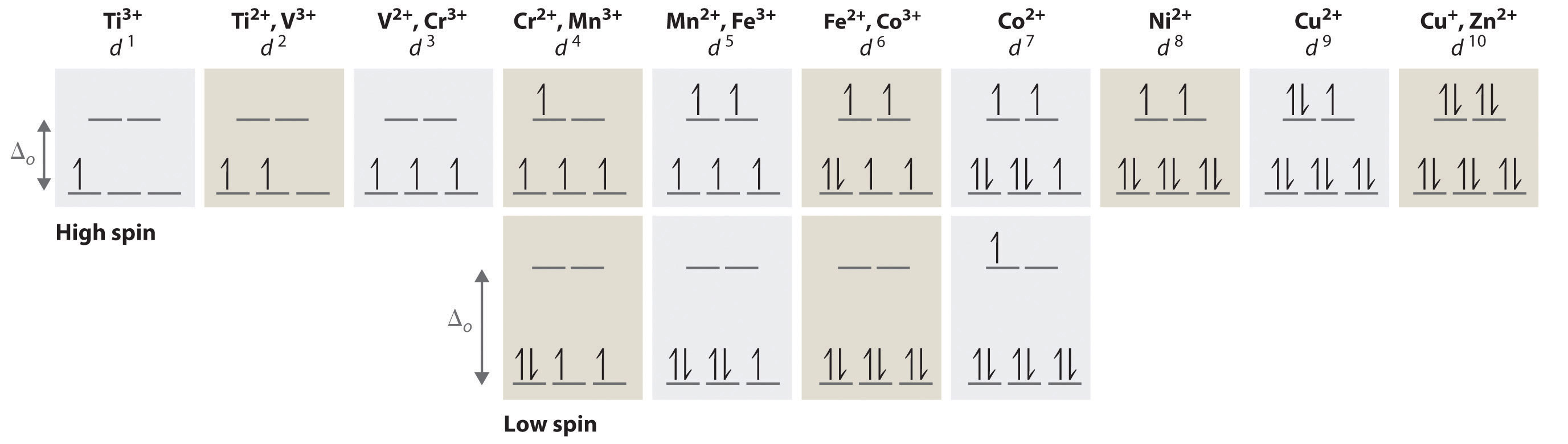
1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2
Periodic Table

Solved Give The Electron Configuration Of Following Ions A Chegg Com
File Hafnium Diagonal Rule Svg Wikibooks Open Books For An Open World
What Is The Number Of Unpaired Electrons In An Fe2 Ion Quora

Electron Structure Of The Atom Ppt Download

