Since that was not done we conclude that the chromium ion was part of a soluble. For instance if you had a 800 g sample of a compound that was 200 g element X and 600 g element y then the percent composition of each element would be.

Solved Which Of The Following Shows The Balanced Equation Chegg Com

Solved Part D Redox Reactions Part I Copper Oxide Chegg Com

Making Salts Soluble Salt Insoluble Salt Acid Excess Insoluble Solid Ppt Video Online Download
High purity Hydrochloric Acid also known as Muriatic Acid is in stock and is ready to be shipped to your home laboratory or business.
Copper oxide and sulfuric acid formula. Another group was exposed for 180 minutes and killed at 0 3 6 12 18 or 24 hours after exposure. Sulfuric Acid Chemical Name. Sulfuric acid H 2 SO 4 is a strong acid with hygroscopic and oxidizing properties.
Sulfuric Acid is a mineral acid with a chemical formula H 2 SO 4. The copper atoms arrange in a fcc. Vinegar is a naturally-occurring liquid that contains many chemicals so you cant just write a simple formula for it.
Armed with a basic Periodic table students can begin to write chemical formulas in one easy lesson. A black solid it is one of the two stable oxides of copper the other being Cu 2 O or copperI oxide cuprous oxide. The chromiumIII ion is presented as an ion meaning its soluble.
If you know the acid formula you will always get the correct anion formula and its charge since the charge is equal to the number of ionizable hydrogen atoms in the acid and is always negative. ChromiumIII sulfate is not soluble which means you would have to write the full formula. 3 Carbonic Acid Cu Copperl cuprous.
This is either sold for industrial use or used to extract oxide ores of copper by leaching. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Oxide is a catalyst for this reaction.
One way of solving the problem is to convert it to sulfuric acid. So there are actually two main chemical formulas involved. Using sulfuric acid can be done but and this is part of the informed prediction probably should not.
In this step the hydrogen ions are taken up by the oxide ion producing water as a product. It has a strong acidic nature and is corrosive. 93 wt sulfuric acid has a freezing point below zero at -21ᵒF so many industrial applications can utilize carbon steel tanks with 93 wt H 2 SO 4 uninsulated but 98 wt freezes at 30ᵒF and so.
It is approximately 5-20 acetic acid in water. So here on adding zinc to CuSO4 solution zinc displaces copper from copper sulphate forms zinc sulphate solution. As a mineral it is known as tenoriteIt is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.
Explanation - Zinc is more reactive than copper. Dilute sulfuric acid and nitric acid produce copperII sulfate and copperII nitrate respectively. This is the quickest and easiest way to learn.
Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid. CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice. The net overall reaction is CuO H 2 SO 4 CuSO 4 H 2 O.
The white sugar turns into a black carbonized tube that pushes itself out of the beaker. Chemical Product and Company Identification Product Name. When it does the sulfate ion remains.
Turn over b Some magnesium powder is added to dilute sulfuric acid in a test tube. Sulfuric Acid 93 CAS. What is Sulfuric Acid.
In an experiment studying the clearance via the blood of radiolabeled sulfuric acid aerosol in different species the authors have observed that sulfur from sulfuric acid was rapidly cleared from 2 to 9 minutes from the lungs of animals into the blood following inhalation exposure. CuSO4 solution has a blue colour while ZnSO4 solu. 4 Sulfuric acid Cr3 ChromiumIII.
Sulfuric acid is also known as Mattling acid or Oil of vitriol. Hydrogen sulfate Chemical Formula. Cu 2 O crystallizes in a cubic structure with a lattice constant a l 42696 Å.
This reaction is an example of an acidbase reaction. Aside from the sulfurous odor the reaction smells a lot like caramel. The basic equation mass of element mass of compound X 100.
CopperII oxide or cupric oxide is an inorganic compound with the formula CuO. When more magnesium is added the reaction continues for a while and then stops. Animals were exposed for 15 30 45 or 60 minutes and killed immediately.
This is indicated by colour change from blue to colourless. The structural formula for acetic acid is CH 3 COOH. Complete these in lab and on your own time for practice.
Chemical Reaction Formula Atomic Mass Formula Chemical Formula Enthalpy Formula Entropy Formula Molality Formula Molar Mass Formula Molarity Formula Structural Formula Molecular Formula Chemical Compound Formula Chemical Equilibrium Formula Normality Formula Photosynthesis Formula Grams to Moles Conversion Formula Moles to Grams Conversion. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Answer 1 of 17.
Sulfate is a normal constituent of the blood and is a normal metabolite of sulfur-containing amino acids and. Calcium copper iron magnesium zinc Metals in dilute. A colourless solution is formed and a gas is given off.
Calculate the empirical formula of an unknown copper oxide CuxOy. Exactly 400 g of a solution of sulfuric acid was diluted with water and excess of CaCl_2 was added. The molecular formula for water is H 2 O.
Cu 2 O degrades to copperII oxide in moist air. Sulfuric acid is a strong acid and in the right circumstances can give up two H ions. Smelting with fluxes The calcine is heated to over 1200 C with fluxes such as silica and limestone.
The freezing point of different sulfuric acid concentrations can vary markedly. Percent composition in chemistry typically refers to the percent each element is of the compounds total mass. The calcine melts and reacts with the fluxes.
Learn how to write the chemical formula of a variety of chemical compounds using the arms and link method. Sulfuric acid 98072 g. The sulfuric acid removes water from the sugar in a highly exothermic reaction releasing heat steam and sulfur oxide fumes.
You can buy Hydrochloric Acid also known as Muriatic Acid online or you can call 512-668-9918 to order your chemicals by phone. Sulfuric Acid 93 Safety Data Sheet SDS Health 3 Fire 0 Reactivity 0 Personal Protection 3 0 Section 1. For example for sulfuric acid H 2 SO 4 the anion is sulfate SO 4 2- with a -2 charge.
The pulmonary uptake of copper oxide occurred in rats exposed to aerosols containing 50-80 mgcu m.

Crystallisation 1 Copper Ii Oxide And Dilute Sulphuric Acid Youtube
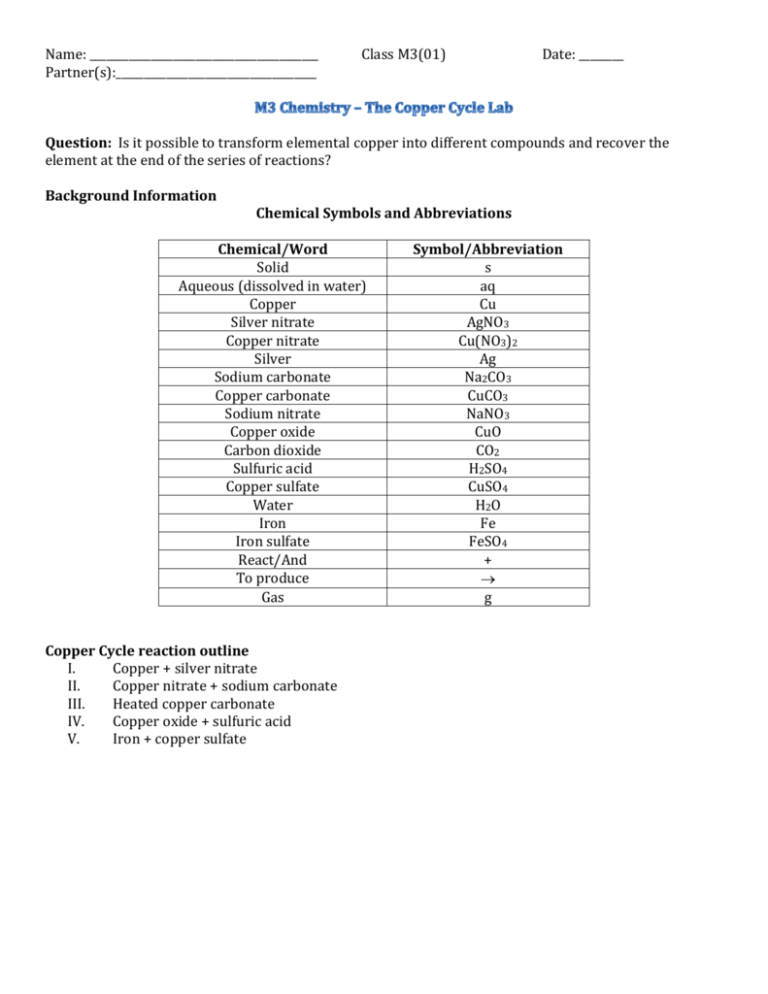
Copper Cycle Lab Instructions

Question Video Reaction Of Sulfuric Acid With Cupric Oxide Nagwa

Metals Revision Properties And Reactions Of Metals Ppt Download
Cuso4

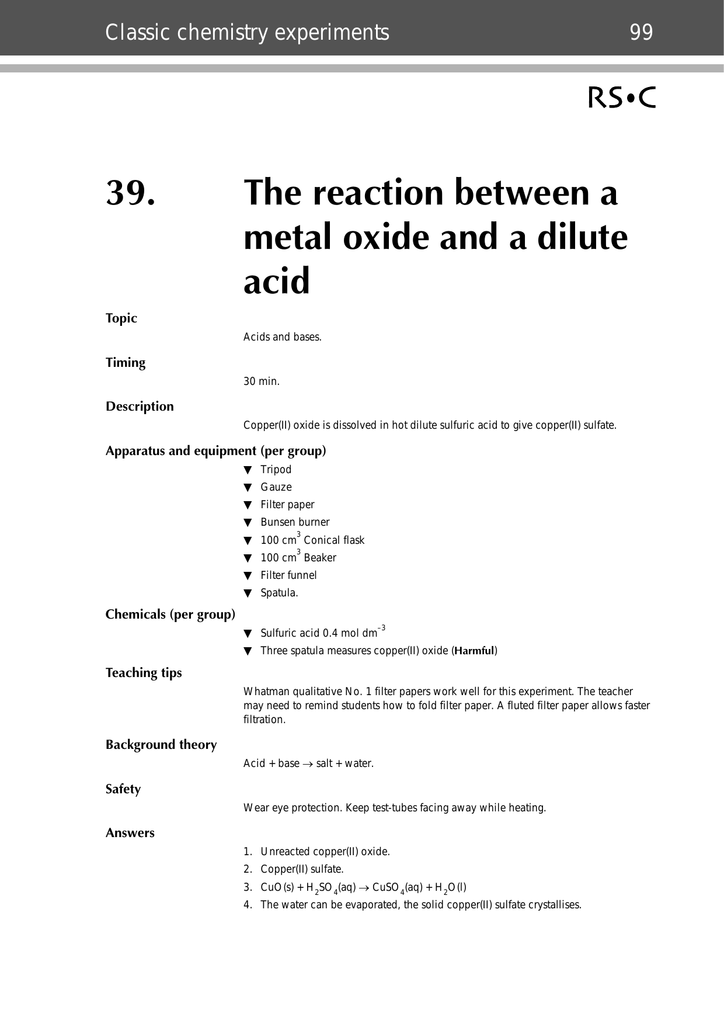
39 The Reaction Between A Metal Oxide And A Dilute Acid

Calculation Of Quantities Required For A Chemical Reaction

1 Chapter 20neutralization And Salts 20 1neutralization 20 2practical Applications Of Neutralization 20 3salts Of Some Common Acids 20 4water Of Crystallization Ppt Download

