NCERT Solutions for Class 12 Chemistry Chapter 8 The d and f Block Elements is a powerful study material that has answers to textbook exercises and important questions from the previous year and sample papers. Lune pour les réactions doxydation et de réduction.

3 1 Electron Configurations Problems Chemistry Libretexts
Quick Answer Why Size Of Zn Is More Than Cu Voip

Write Down Electronic Configuration Of Cr3 And Cu2 Ions Calculate The Number Of Unpaired Electrons Brainly In
Electronic configuration of an element.
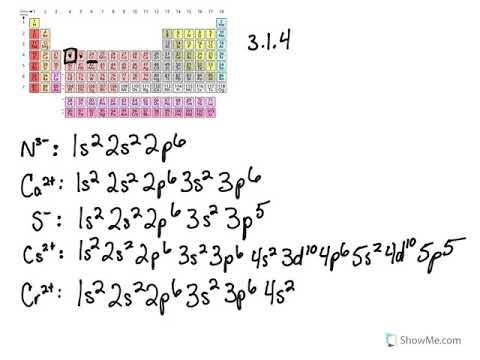
Cu2+ electron configuration. Iii Elements with this electron configuration are nonmetals. Log in to access Animations preview Accessing the animations Assigning quizzes Viewing students scores Order form Free Biology animations Breathing animation Fertilisation animation Plant and animal cells The Ear animation Starch in leaf test animation Kidney animation DNA drag and drop Alveoli animation Blood clotting animation. The electron configuration and the orbital diagram are.
Consider the general valence electron configuration of ns2np5 and the following statements. ˈ r ɛ d ɒ k s RED-oks or ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of atoms are changed. I know ill need a 2p orbital but theres orbital mixing going on so i have 2 choices.
It is very difficult to remove an electron from this arrangement. Chemistry Notes for Students. Dans la méthode ion-électron également connue comme une méthode des demi-réactions léquation redox est divisée en deux équations aux dérivées partielles.
Preparation and properties of KMnO 4 and K 2Cr 2O 7. This electron configuration of Mo is in the condensed form and on the ground state. A Lewis acid named for the American physical chemist Gilbert N.
To write the configuration for the Copper ions first we need to write the electron configuration for just Copper Cu. Rules for filling electrons in orbitals Aufbau principle Pauli exclusion principle and Hunds rule electronic configuration of elements the extra stability of half-filled and completely filled orbitals. Shapes of s p and d orbitals electron spin and spin quantum number.
Excited state electron detachment ESED and phtoaquation reactions were clarified by comparing the results of 260 320 340 and 350 nm excitations. His main research interest focuses on the optical. Chacune de ces demi-réactions séquilibre séparément et après elles se somment pour donner une équation redox équilibrée.
We first need to find the number of. Shapes of s p and d orbitals electron spin and spin quantum number. Cu2Cu with change in concentration of electrolytes CuSO4 or ZnSO4.
Electron affinity measures the tendency of an element to form an anion. SECONDARY STAGE CHEMISTRY BOOK ONE FOR CLASS IX. Rules for filling electrons in orbitals aufbau.
Redox reactions are characterized by the actual or formal transfer of electrons between chemical species most often with one species the reducing agent undergoing oxidation losing electrons while. Following hydrogen is the noble gas helium which has an atomic number of 2. The reversibility of the system allows for the flavylium cation to be recovered by other external stimuli completing one cycle.
Cu2 SO 2 2-H OH-. Dr Li is currently a Professor at School of Materials Energy previously at School of Physical Science Technology Lanzhou University China. We would like to show you a description here but the site wont allow us.
Academiaedu is a platform for academics to share research papers. Unit 4 Chemical Bonding and Molecular Structure. 2 Structure of Atom Discovery of Electron Proton and Neutron atomic number isotopes and isobars.
The overall trend in electron affinities is to increase from left to right across a period. In a second titration50cm3 aliquot treated with hekamethylenetetraamine to mark chromium requires 3543cm3 of 0058M EDTA to reach the end point. Side by Side Comparison.
The electron in the orbit nearest the nucleus has the lowest energy. Similarly to metamorphosis in biology a molecule flavylium cation generates a sequence of other different molecules by means of external stimuli. Ii Elements with this electron configuration are expected to have large positive electron affinities.
In general electro negativity decreases down the Group as successive energy levels electron shells. Lewis is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adductA Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis. NCERT Solutions for Class 12 Chemistry Chapter 8 Free PDF Download.
To list the elements order by ionization energy click on the table headers. Footprints-Science - GCSE science animations and quizzes. These NCERT Solutions for Class 12 Chemistry are updated to the latest term II CBSE Syllabus for 2021-22 and.
The electron affinity is the energy change for the addition of 1 mol of electrons to 1 mol of gaseous atoms or ions. Finally a third 50cm3 aliquot was heated with 50cm3 of 0058molar EDTA and back titrated with 625cm3 of 0063M CU2 to reach d end point with murroxide indicator. Which one of the following sets of ions represents a collection of isoelectronic species.
I Elements with this electron configuration are expected to form -1 anions. Which is a very stable electron configuration. A charge-transfer CT complex composed of rac-33-dibromo-11-bi-2-naphthol as an electron donor and 11-dimethyl-44-bipyridinium dichloride as an electron acceptor serves as a host.
How to Write the Electron Configuration for Copper Cu Cu and Cu2 In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom there are 29 electrons. Ion charges worksheet answers. Because According to molecular orbital theory O 2 has 15 electrons it has one electron in antibonding orbital.
Various quantum numbers principal angular momentum and magnetic quantum numbers and their significance. The chemical symbol for Rubidium is Rb. Wiki User Answered 2012-03-05 064157.
Configuration oxidation states and comparison with lanthanoids. Once we have the configuration for Cu the ions are simple. In this study the excited-state dynamics of K4FeCN6 were studied by transient absorption spectroscopy.
Elementary ideas of quantum mechanics quantum mechanical model of atom its important features concept of atomic orbitals as one electron wave functions. The electronic configuration for oxygen is written as 1s2 2s2 2p4.

What Is The Electronic Configuration Of Copper Cu Cu And Cu2

Solved Write The Electron Configuration Of Cu2 1 B What Chegg Com

Write The Electronic Configuration Of Cu2 Ion Z 29 Brainly In

Webelements Periodic Table Copper Properties Of Free Atoms
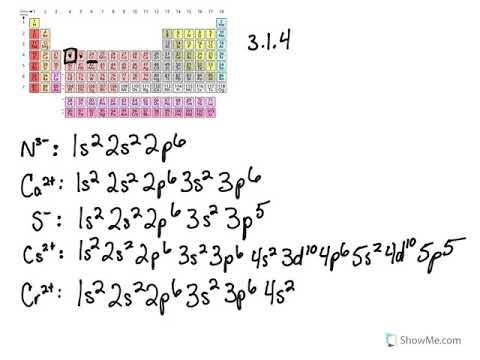
3 1 Electron Configurations Problems Chemistry Libretexts

3 1 Electron Configurations Problems Chemistry Libretexts
The Electronic Configuration Of Cu 2 Ion Is
Why Do Cu Fe 3 And Mn 2 Have Colored Solutions Despite Having Half Filled And Full Filled D Orbitals Socratic

