The H-H bond requires 436 kJmol to break and the Cl-Cl bond requires 242 kJmol. Use bond energies to estimate enthalpy.
Solved Use Bond Energies To Calculate The Heat Of Reaction Chegg Com
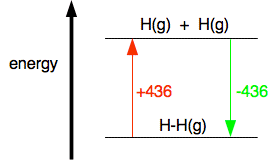
Bond Enthalpy Bond Energy
Chemical Bond Energy Example
Tools for comparing experimental and computational ideal-gas thermochemical properties.
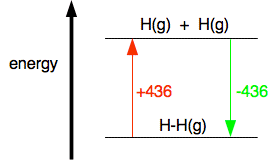
Bond enthalpy calculations. And for sodium chloride negative 40727. The standard enthalpy of formation for hydrochloric acid is negative 1672 kilojoules for mole. Elements may be in any order.
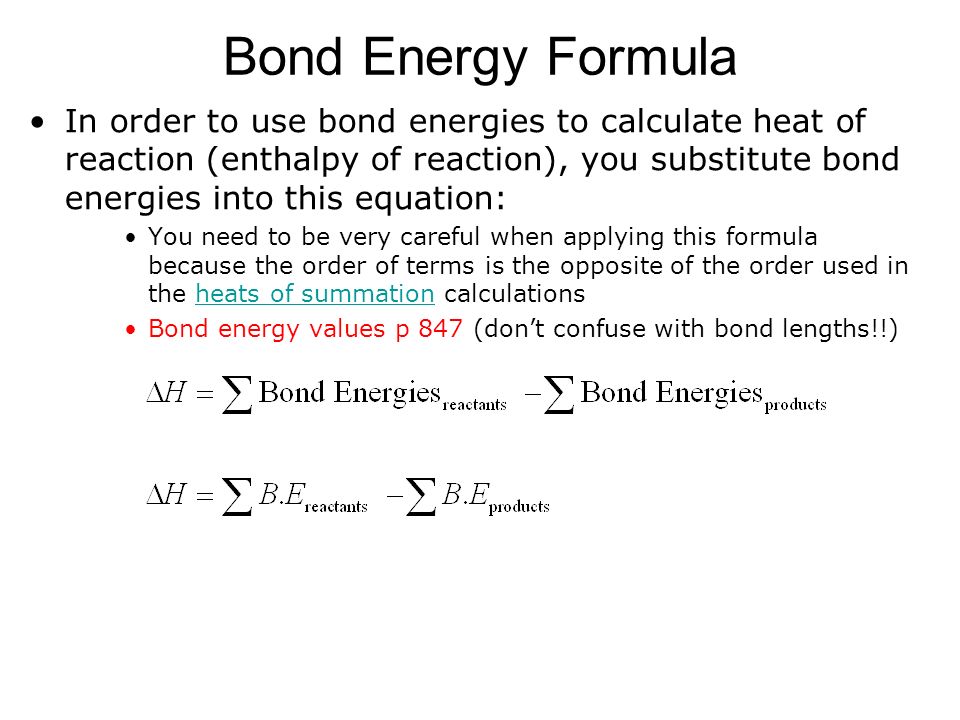
You cannot use bond enthalpies to do calculations directly from substances starting in the liquid or solid state. The standard enthalpy of a bond is the enthalpy required to break the bond usually at 25C and 1 atm. The standard enthalpy of reaction latexDelta Hominus _rxnlatex is the change in enthalpy for a given reaction calculated from the standard enthalpies of formation for all reactants and products.
Anion enthalpy of formation from the electron affinity and radical enthalpy of formation. Here is a website with lots of practice quizzes on all three types of enthalpy calculations. Enter a sequence of element symbols followed by numbers to specify the amounts of desired elements eg C6H6.
So even though enthalpy does depend on temperatures ignoring the effect doesnt usually have a big impact on calculations. Enthalpy changes for a phase change so the enthalpy of a substance depends on whether is it is a solid liquid or gas. Nearly all chemical reactions involve forming or breaking bonds between atoms.
A graphic analysis of these calculations. Im not gonna do the mole calculations on screen. For liquid water negative 2858.
Note that most of the time the values at the two temperatures are not significantly different. Definition Calculations Values. It is a state function used in many measurements in chemical biological and physical systems at a constant pressure which is conveniently provided by the large ambient atmosphere.
In about a month once we have learned how to draw Lewis structures you. Hydrogen peroxide water. The pressurevolume term expresses the work.
And so if a chemical or physical process is carried out at constant pressure with the only work done caused by expansion or contraction then the heat flow q p and enthalpy change ΔH for the process are equalThe heat given off when you operate a Bunsen burner is equal to the enthalpy change of the methane. The ΔH for this reaction is -184 kJmol. ΔH is the change in Enthalpy.
This lesson covers the following objectives. Use bond energies to determine the energy change for the following reaction. Overall the reaction will release 184 kJmol.
Standard enthalpy of reaction. This type of bond energy does not apply to ionic bonds. Methane oxygen carbon dioxide water.
To learn more about how to calculate the energy needed to break bonds review the corresponding lesson on Bond Enthalpy. Can be used to determine the bond energy. Enthalpy ˈ ɛ n θ əl p i a property of a thermodynamic system is the sum of the systems internal energy and the product of its pressure and volume.
As an example of bond dissociation enthalpy to break up 1 mole of gaseous hydrogen chloride molecules into separate gaseous hydrogen and chlorine atoms takes 432 kJ. Where q p is the heat of reaction under conditions of constant pressure. Enthalpy is the amount of heat energy possessed by substances.
Rules for chemical formula. Machine learning predictions of bond dissociation energies BDEs This tool predicts BDEs for single noncyclic bonds in neutral organic molecules consisting of C H O and N atoms. The bond dissociation enthalpy for the H-Cl bond is 432 kJ mol-1.
Determine the enthalpy change for the following reaction. Mean absolute errors are typically less than 1 kcalmol for most compounds. When 2 atoms bind together to form a new molecule it is possible to determine how strong the bond between atoms is by measuring the amount of energy needed to break that bond.
Bond Dissociation Energy from the enthalpy of formation of the acid and radical. Enthalpy of formation of the acid from its Bond Dissociation Energy and the enthalpy of formation of the radical. Since in a chemical reaction energy can be neither destroyed nor created if we know the energy required to form or break the bonds being made or broken in the reaction we can estimate the enthalpy change for the entire reaction with high accuracy by adding up.
AUS-e-TUTE is a science education website providing notes quizzes tests exams games drills worksheets and syllabus study guides for high school science students and teachers. Calculations of this type will also tell us whether a reaction is exothermic or endothermic. Similar calculations lead to a value of 2902.
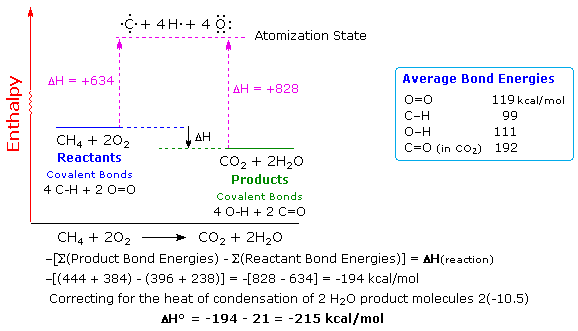
We can do this again by using the average bond enthalpies of C-H CO OO and O-H bonds. Be sure to specify the phase of the reactants and products using s l or g and be sure to look up the. Bond enthalpy is used for thermochemistry.
H is the Enthalpy. In the above formula the ΔH is obtained by adding up all the di-nucleotide pairs enthalpy values of each nearest neighbor base pair. Bond energy is an important concept in chemistry that defines the amount of energy needed to break a bond between a covalently bound gas.
C2H4g F2g C2H4F2g Determine the energy change for the following reaction. For sodium hydroxide its negative 46915 kilojoules per mole. To use enter a SMILES string above or use the drawing tool and press submit.
A specific example can be made from our old familiar combustion of methane reaction. Here In Conclusion at this point we have learned 3 ways to calculate the Heat of a Reaction. The enthalpy change that occurs in a system when one mole of matter is transformed by a chemical reaction under standard conditions.
We calculated the enthalpy change during this transformation before from traditional thermochemcial methods. When the two H-Cl bonds form they release 862 kJmol 431 from each bond. The enthalpy of vaporization symbol H vap also known as the latent heat of vaporization or heat of evaporation is the amount of energy that must be added to a liquid substance to transform a quantity of that substance into a gasThe enthalpy of vaporization is a function of the pressure at which that transformation takes place.
In other words if a chemical change takes place by several different routes the overall enthalpy change is the same regardless of the route by which the chemical change occurs provided the initial and final condition are the same. Examples of enthalpy changes include enthalpy of combustion enthalpy of fusion enthalpy of vaporization and standard enthalpy of formation. The total enthalpy input is 678 kJmol.
The law states that the total enthalpy change during a reaction is the same whether the reaction is made in one step or in several steps. Bond dissociation energy is favored for theoretical work models and computations. Experimental and computed quantum mechanics thermochemical data for a selected set of 2069 gas-phase atoms and small molecules.
Using the standard enthalpy of formation data in Appendix G calculate the bond energy of the carbon-sulfur double bond in CS 2. The standard enthalpy of formation latexDeltaH_textfcirclatex is the enthalpy change accompanying the formation of 1 mole of a substance from the elements in their most stable states at 1 bar standard state. Assuming the initial molecule and its fragments to be perfect gases the following formula can be written for the difference between enthalpy and energy.
Coefficients refer to the number of molesThus for the first equation -2828 kJ is the ΔH when 1 mol of H 2 O l is formed from 1 mol H 2 g and ½ mol O 2. For example in the reaction in Figure 7-13 two bonds break.
U3 S3 L4 Bond Energies Pages Using Bond Energies Ppt Download
Chem 101 Lecture 17
Chemical Reactivity

Bond Enthalpy Calculations Ib Chemistry Sl Revision Notes
1

Chem 101 Using Bond Energies To Calculate Change In Enthalpy For A Reaction Youtube

Bond Energy Definition Equation Calculations Study Com

Bond Enthalpy Energy Calculations For Chemical Reactions Calculation Of Theoretical Heat Energy Tranfers For Exothermic Or Endothermic Reactions Gcse Notes Ks4 Science Igcse O Level Chemistry Revision

